
- anti-PF4/Heparin IgG antibody with human Fc fragment,
- induces heparin- and FcϒRIIA-dependent platelet activation,
- specific to the PF4/Heparin complex with very low reactivity against PF4 alone.
5B9 clinical research on HIT functional assays covers the following objectives:
- identification of “good” or “bad” platelet donors responders, [2]
- evaluation of platelet reactivity, [2]
- preparation of positive samples. [3]
5B9 may be also used as a specific tool when studying FcϒR-dependent cell activation in the pathophysiology of HIT. [1]
Kits are available in stock to order
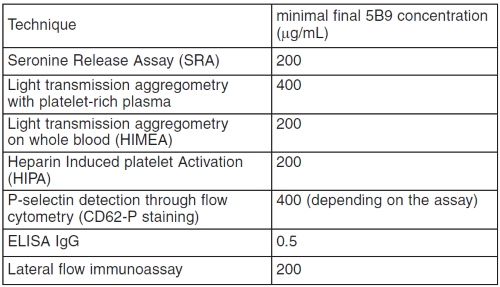
The following table summarizes the minimal final 5B9 concentration in the sample for which each assay gives positive results:
1. KIZLIK-MASSON C., VAYNE C., MCKENZIE S.E., POUPON A., ZHOU Y., CHAMPIER G. POUPLARD C., GRUEL Y., ROLLIN J.: “5B9, a monoclonal antiplatelet factor 4/heparin IgG with a human Fc fragment that mimics heparin-induced thrombocytopenia antibodies”. J.Thromb. Haemost., 15, 2065-2075, 2017.
2. VAYNE C., GUERY E.A., CHARUEL N., BESOMBES J., LAMBERT W.C., ROLLIN J., GRUEL Y., POUPLARD C.: “Evaluation of functional assays for the diagnosis of heparin induced thrombocytopenia using 5B9, a monoclonal IgG that mimics human antibodies”. J. Thromb. Haemost., 18, 968-975, 2020.
3. Can 5B9, a monoclonal anti-PF4/heparin IgG, be used as an internal quality control for HIT platelet functional assays? Présentation Y Gruel SSC Platelet Immunology ISTH 2020.

