As part of Stago's continuous improvement commitment, STA®-ImmunoDef* intrinsic factor kits were developed as a new generation of Deficient Plasmas with enhanced performances.

Intrinsic factor measurement allows for the characterisation of clinically significant conditions such as Haemophilia A (Factor VIII) and B (Factor IX). Robust and reliable reagents promote increased laboratory efficiency. * Availability depending upon the country
Optimum analytical performance for even more reliable patient results
- Extended working range with one calibration curve:
- STA®-ImmunoDef VIII : 0,7-400%
- STA®-ImmunoDef IX : 0,7-300%
- STA®-ImmunoDef XI : 1-200%
- STA®-ImmunoDef XII : 3-300%
- No more low calibration curve needed
- Perfect correlation of low patient results
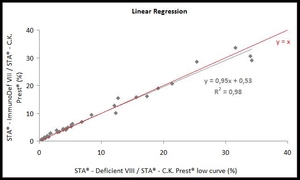
- Very good precision on the entire working range
Adapted reagents whatever the clinical context, especially for the diagnosis and monitoring of haemophilia
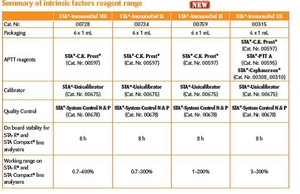
- Enhanced reagents on board stability (8 h)
- - Assay available for a long time
- Stability and robustness of calibrations
- - No systematic calibration needed
Efficient reagents for an easier laboratory practice
Compliant with current international guidelines
- Calibrators & controls assayed against WHO International Standards*
- - For standardised results
- Residual activity of immunodepleted factor <1%*
- - Ensures reliability of patient's for low results
- Other coagulation factors at normal levels
- - For specific measurements
- Addition of VWF (STA®-ImmunoDef VIII)
- - Adapted to Bethesda and Nijmegen inhibitor assays
*STA®-ImmunoDef VIII, STA®-ImmunoDef IX, STA®-ImmunoDef XI
