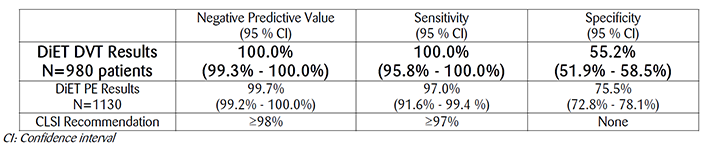
With a Negative Predictive Value (NPV), sensitivity and specificity of 100%, 100% and 55.2% for DVT exclusion and 99.7%, 97.0% and 75.5% for PE exclusion, respectively, STA-Liatest D-Di demonstrates best-in-class clinical performance of automated D-Dimer assays for exclusion of DVT and PE within the rigorous requirements of a comprehensive, prospective management study compliant with CLSI H59-A, Quantitative D-Dimer for the exclusion of venous thromboembolic disease, approved guideline 2011.
For more than 15 years, STA-Liatest D-Di has been the standard of care for quantitative, automated D-Dimer assays in thousands of hospitals, medical centers and private laboratories with more than 2 million patient results reported worldwide. D-Dimer results are critical to the diagnostic algorithm for the exclusion of Venous ThromboEmbolism (VTE) in low and moderate pre-test probability patients. Use of D-Dimer results in VTE patients is guided by CLSI Document H59-A, Quantitative D-Dimer for the exclusion of venous thromboembolic disease, approved guideline published in 2011. To demonstrate reliable clinical performance and compliance with the robust requirements of the CLSI guideline, Stago conducted a 5-year, multi-national, prospective management study, including pre-test probability assessment, objective imaging results and 3-month patient follow-up: the DIET study, registered with the US National Institutes of Health (NCT01221805).
More than 2000 outpatients were enrolled in the DiET study with a suspicion of DVT or PE in 16 sites throughout the world: United States (9), Canada (1), France (2), Italy (2) and Spain (2). Results exceed FDA and CLSI performance goals required for Sensitivity and Negative Predictive Value (NPV), the two most relevant criteria for exclusion of DVT and PE in patients with low or moderate pre-test probability as stated in the CLSI H59-A.
STA®-Liatest® D-Di also demonstrated best-in-class clinical specificity in the DiET study. Higher specificity indicates fewer false positive D-Dimer results and fewer unnecessary referrals for imaging studies that may increase the cost of patient care, increase length of stay and increase patient exposure to radiation and associated complications of imaging studies.