
In the US, Xarelto is approved for stroke prevention in AF patients and prevention of VTE in hip or knee surgery.
In total, Rivaroxaban is approved in more than 110 countries worldwide. This makes Rivaroxaban a future major player in the anticoagulants world.
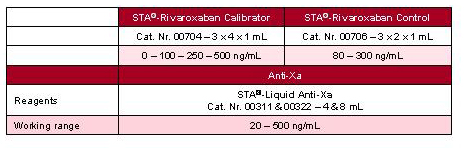
STA®-Rivaroxaban Calibrator & Control is a solution for the determination of Rivaroxaban concentration to support the examination of patients in different clinical situations.
Including Rivaroxaban calibrators and controls, this solution uses an anti-Xa method (STA®-Liquid Anti-Xa), insensitive to analytical and biological variables, with a wide working range.
Results are expressed in ng/mL of Rivaroxaban [1]
Reagents are offered with all the standard guarantees:
- barcoded reagents for optimal ease of handling and traceability
- results available in a few minutes only, adapted to STAT samples requirement
- fully automated on STA-R® and STA-Compact®, with dedicated tests setups
1. SAMAMA M.M. et al., Evaluation of the anti-factor Xa chromogenic assay for measuring rivaroxaban plasma concentrations using calibrators and controls, ASH, 5071 b JN04, Abstract, 2010.
*Note: Not available in all countries. In the USA this product is classified as "For Research Use Only. Not for use in diagnostic procedures"
